
Modernization of the ClinicalTrials.gov Protocol Registration and Results System and ClinicalTrials.gov Website
Do you have ideas about how to improve the ClinicalTrials.gov user experience? Now is the chance to give your opinion.
The ClinicalTrials.gov Protocol Registration and Results System (PRS), where researchers register studies and report results information, and the public facing ClinicalTrials.gov website are both being updated to improve the user experience. Test sites, or beta release websites, of the ClinicalTrials.gov PRS website and the publicly accessible ClinicalTrials.gov website, are available for use and there is an opportunity to provide feedback during the redesign process.
Goals and Implementation
The ClinicalTrials.gov modernization effort seeks to improve the user experience with new and updated websites that will be easier and faster to use.
The goals of the updated test sites, or beta releases, of the ClinicalTrials.gov PRS and ClinicalTrials.gov website and components are to:
- Introduce users to the new technology platforms and evaluate their real-world performance.
- Provide ClinicalTrials.gov PRS users with improved methods to manage their ClinicalTrials.gov records.
- Offer new foundational features that will be expanded over time.
- Collect user input to inform future development.
The beta sites currently function in parallel to the existing systems to allow for user testing and improvements.
Beta ClinicalTrials.gov PRS Release
Updates in the new ClinicalTrials.gov PRS Release (PRS Beta) are now available at register.clinicaltrials.gov/beta. A recorded demonstration to preview ClinicalTrials.gov PRS Beta is available.
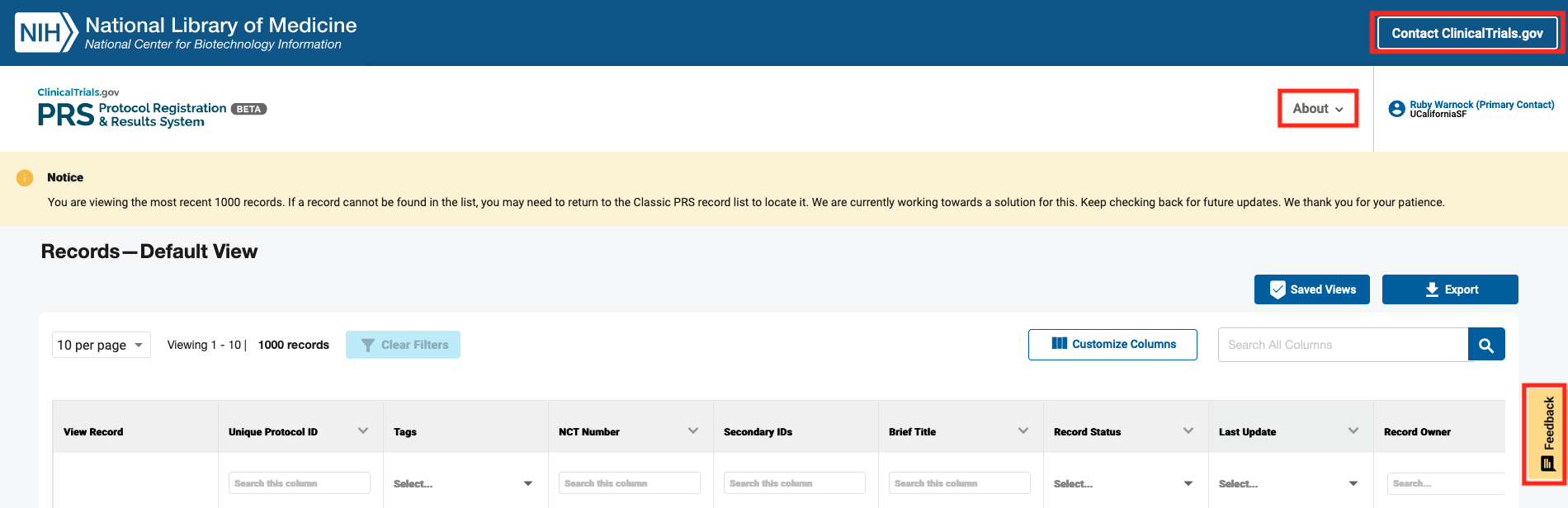
The ClinicalTrials.gov PRS Beta release includes:
- New workflow and management features such as a customizable display and multiple download options to help users save time.
- Improvements to the functionality of the Record List.
- An option to click on buttons to contact ClinicalTrials.gov with questions, give feedback about PRS Beta, and learn about PRS Beta:
- A pop-up window was added to the Contact ClinicalTrials.gov button to provide other options for finding answers to questions and getting help with using PRS Beta.
- To provide feedback, click on the yellow feedback button on the bottom right side of the webpage.
- An About menu was added that includes links to the About PRS Beta page and the release notes.
Beta ClinicalTrials.gov Release
The new updated public facing beta ClinicalTrials.gov homepage is now available by clicking on the banner link on Home – ClinicalTrials.gov or directly via beta.clinicaltrials.gov.
The Release Notes Page has more information about current and future updates.
The modernization effort implements a responsive website design that better supports mobile device users and adds information written in plain language. There are multiple ways to provide feedback as you explore the beta website.
Measuring Success, Next Steps, and Future Releases
User feedback received from website surveys, analytics, and continued engagement will help Clinicaltrials.gov PRS staff to assess the success of the first beta releases. Specific details of each component and release will be available.
To learn about related events and upcoming website enhancements and features, visit the ClinicalTrials.gov Modernization webpage.
For ClinicalTrials.gov PRS email updates, sign up for Hot Off the PRS.
If you have specific topics or questions you would like to see addressed in future ClinicalTrials.gov Regulatory Support Newsletter editions, please notify the UCSF ClincialTrials.gov Regulatory Support Team, Ruby Warnock, and Melanie Hassel. An archive of the ClinicalTrials.gov Regulatory Support Newsletter is available on the UCSF Clinical Research Resource HUB.
Please reach out to the UCSF ClincialTrials.gov Regulatory Support Team if you have anything to discuss relating to ClinicalTrials.gov. We are always happy to hear from you.
If you are a researcher affiliated with the Helen Diller Family Comprehensive Cancer Center, please contact Julie Robbins with the UCSF Helen Diller Family Comprehensive Cancer Center Data Integrity and Research Compliance Team for assistance with ClinicalTrials.gov.